If you have been in business for a few years, you already have a Quality Management System (QMS), but it might be informal, inconsistent, or incomplete. If you want to develop it, there are no shortcuts. Fortunately, there is an approach to setting up a quality management system that has proven effective in China and other countries.
What Is A Quality Management System in Manufacturing?
Simply put, a QMS is a way a company ensures it delivers what it promises. It is not just a set of documents or a state of mind; it requires many elements that pull the factory in the same direction and helps different parts support each other.
What Is A Quality Management System Example?
- A system that has a few high-level goals (e.g. customer satisfaction).
- Some measurable and specific objectives that support the higher-level goals (e.g. ensuring at least 90% of customers rate the company’s offer at least 3 stars out of 5)
- A system with a certain way of working, and associated training and coaching programs to achieve the objectives (e.g. standard operating procedures, an employee onboarding program, certification).
They don’t necessarily all have to be documented. The excessive focus on having all documents in order comes from standard auditing practices, not from the needs of the quality management system itself.
3 Steps for Setting Up an Effective QMS for Manufacturing
Step 1: Map Out Your Processes & Controls
This aspect is often forgotten, yet it is one of the few essential steps in setting up a quality management system.
Draw a map of all the process steps. It will help you notice inefficiencies and to propose changes. For example, specific steps might be combined, semi-automated, or eliminated. Usually, the fewer steps, the better, since hand-offs from one team or department to another tend to generate mistakes and delays.
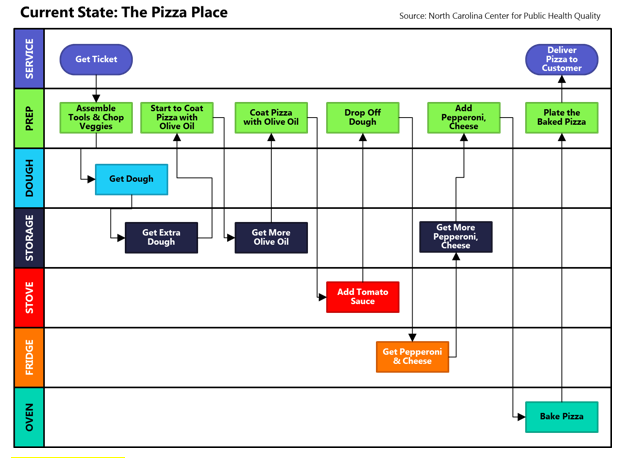
Source: North Carolina Center for Public Health Quality
Once you have listed your processes, you need to determine those that are ‘critical to quality’. Those important to control as a second priority, and finally, those that don’t need to be controlled formally.
Step 2: Create Documentation for Your Procedures & Protocols
Certain documents are apparent – for example, work instructions and product specification sheets for the production and the quality staff. You can’t skip on that. And don’t hesitate to try new things – videos are usually much better than black-and-white sheets of paper full of text.
You will need a document control procedure. Without it, employees might use the wrong version of a document – for example, an out-of-date drawing from a customer. Developing a new product should be tied closely to your engineering change request procedure.
Step 3: Continuously Refine Your Manufacturing Operations & Procedures
SO 9001 contains several clauses about staff competency, so quality managers know training is necessary. However, some essential steps in setting up a quality management system are overlooked by 99% of Chinese factories.
What about maintenance? Making sure your equipment is up and running in good conditions strongly impacts quality and costs. If a tool gets used up, it might damage parts that have to be scrapped later. And yet, preventive and predictive maintenance is often a blind spot.
Regularly reviewing testing procedures is also often overlooked. People use methods written 5 years ago and never wondered if they are still adequate. Very often, we find a few testing protocols that make no sense.
The Bottom Line
A quality management system helps you cut costs. It is not just about pleasing customers, and it is not primarily about documentation but about process control and risk reduction. And, when done well, about process improvement.
What about you? Have you got experiences to share about setting up a quality management system for your company, or your suppliers? Any positive points? Please leave a comment below and we will respond.





